Does The World Have Enough Lithium For Batteries?
Fossil fuels have controlled the global energy supply for many years. Still, many consider it as “the most systematic threat to humankind”.
Fossil fuels have controlled the global energy supply for many years. Still, it has come at the cost of environmental pollution, which is considered “the most systematic threat to humankind” by the United Nations.
Why Sodium-Sulfur Batteries Are The Future
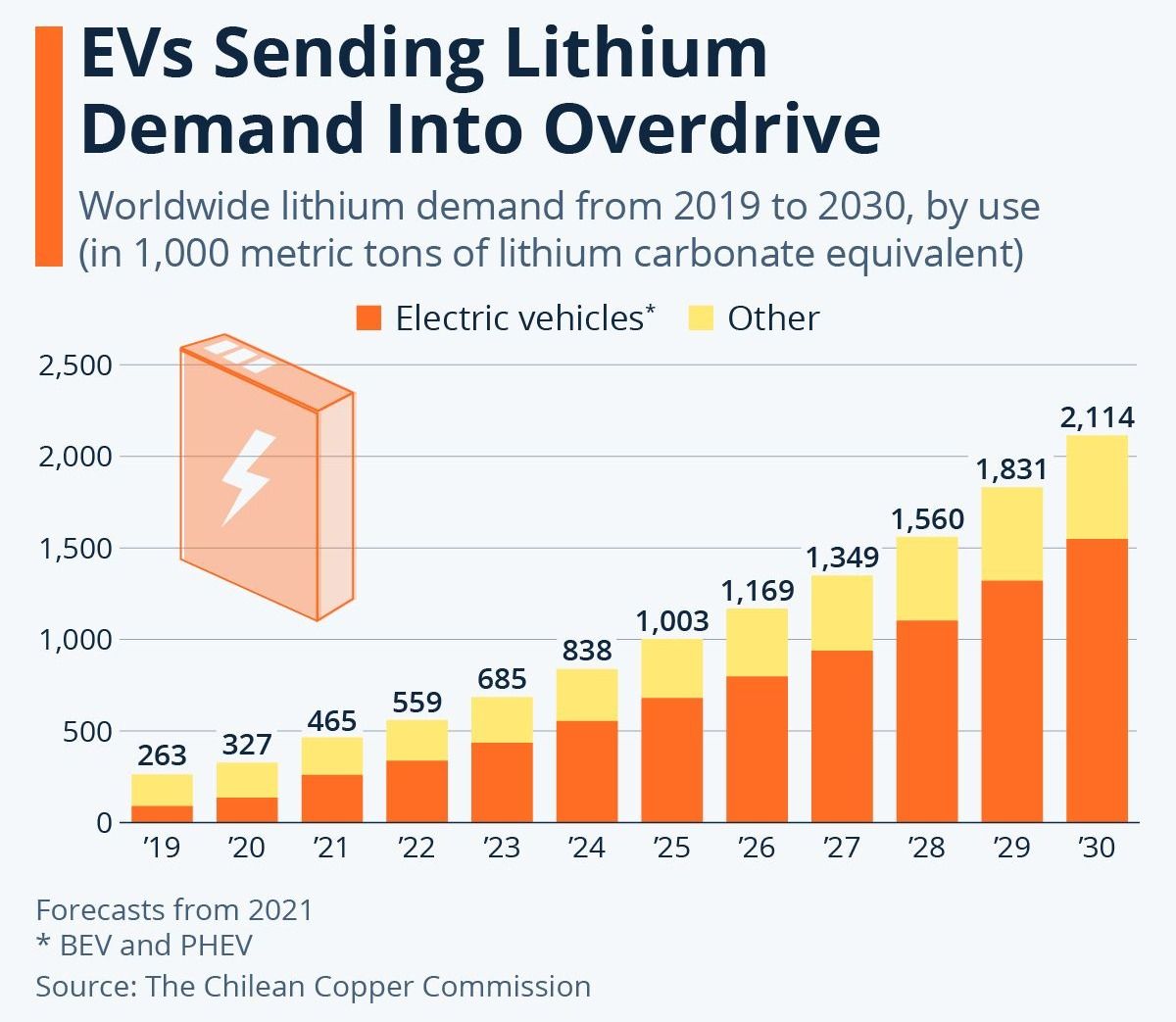
In recent years, the automobile industry and consumer technology’s rising pursuit of environmentally friendly battery storage units has caused an exponential increase in demand for lithium-ion batteries. However, at the current exponential consumption of lithium, a shortage of lithium-ion batteries is expected to occur by 2025 as lithium is a non-renewable source. Many companies know lithium’s lack of supply and are searching for lithium-ion battery alternatives. In particular, sodium-sulfur rechargeable batteries have been gaining attention due to sodium’s abundance in nature and the battery’s environmental friendliness.
How Does Sodium-Sulfur Batteries Work?
A sodium-sulfur battery is a molten-salt battery constructed in a cylindrical configuration. Its top is sealed with an airtight lid to protect the sodium from water and oxidizing atmospheres. The battery is enclosed by a steel casing coated with chromium or molybdenum to protect the interior from corrosion.
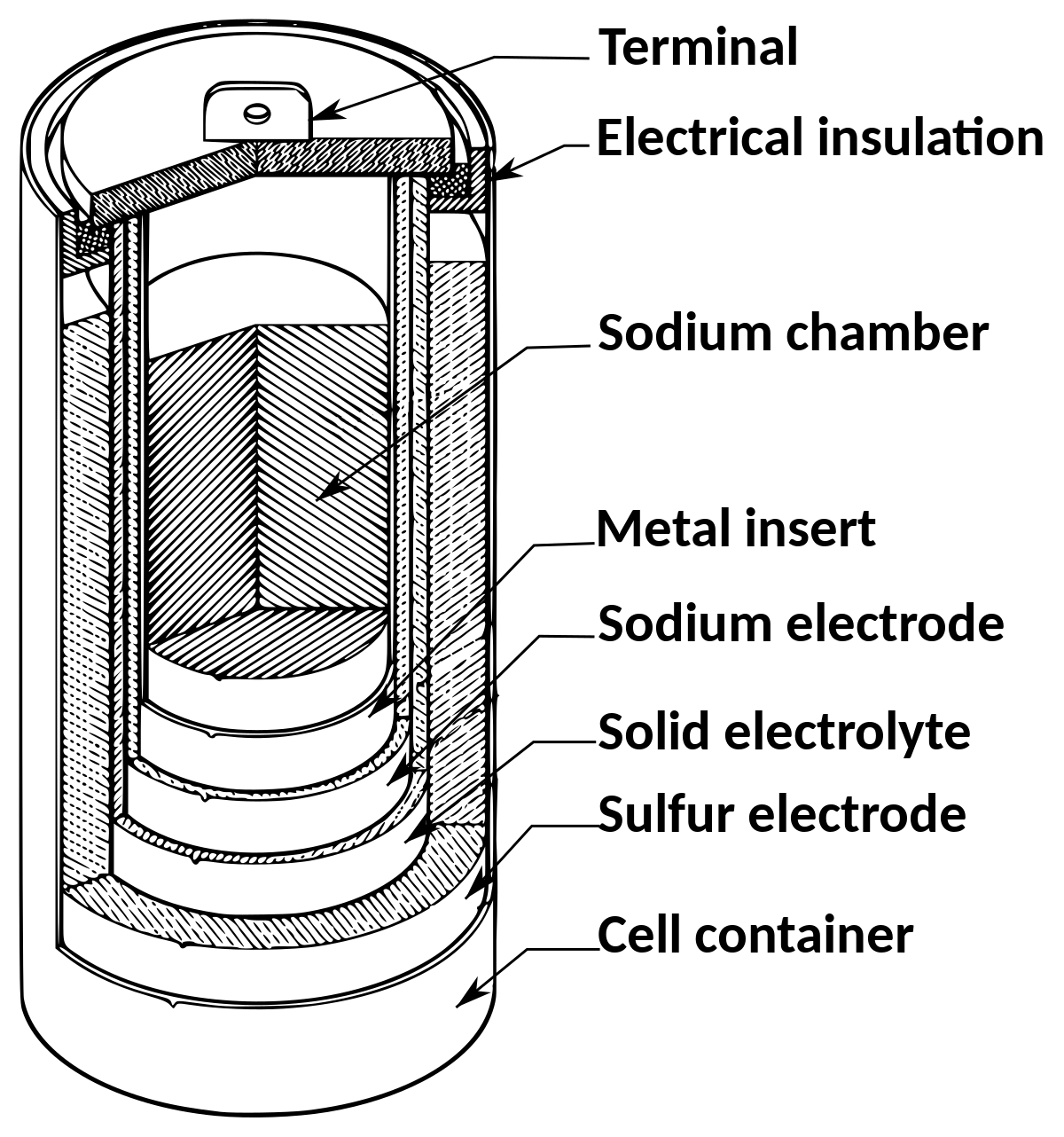
During discharge, electrons are stripped off the sodium, and one electron from each sodium atom becomes sodium ions. The sodium ions travel through the solid electrolyte to the sulfur electrode, carrying an electric current of around two volts to the electric load. At the sulfur electrode, it reacts with the sulfur to form sodium polysulfide. The battery’s high conductivity is obtained at 300°C to keep the elements molten, as ions cannot flow through solids. During charge, the process is reserved so sodium ions are released from the sodium polysulfides to recombine as elemental sodium. Thus, a discharge and charge cycle is created to produce electrical energy.
The Environmental Advantages
Sodium-sulfur batteries have environmental advantages similar to lithium-ion batteries, but what makes them unique to other alternatives is the element’s abundance and rechargeability. The quantity of sodium and sulfur to lithium in the Earth’s crust is 23,600 ppm and 350 ppm to 20 ppm. Hence, although sodium and sulfur’s demand will not meet the supply anytime soon, it is inevitable as all Earth minerals and metals are non-renewable.

However, sodium-sulfur batteries reduce disposable batteries’ waste as they can last 4500 cycles. When batteries corrode in landfills, they release toxic metals such as cadmium and lead, contaminating the topsoil, groundwater, and air. Cadmium is a human carcinogen, while information contributes to neurological damage and cognitive disabilities. With a foreseeable shortage of lithium, sodium-sulfur batteries retain the environmental benefits of lithium-ion batteries and act as a long-term solution due to sodium and sulfur abundance.
The Social Disadvantages
However, sodium-sulfur batteries are challenged by their social disadvantages over lithium-ion batteries. Many safety concerns are associated with the battery, as sodium and sulfur are both reactive elements. For example, sodium has a violent reaction in contact with water. The response is highly exothermic and explosive; the products are hydrogen gas and sodium hydroxide. Hydrogen is highly flammable and combustible in the presence of oxygen; sodium hydroxide is poisonous if ingested and can cause severe chemical burns.
Moreover, sulfur dioxide is produced by the combustion of sulfur, toxic gas, and a tumorigen. Although safety precautions are implemented in the battery’s design, sodium-sulfur batteries have only recently attracted global attention. After the storm exploded in the Tsukuba Plant in 2011, the relatively new technique of its safety systems imposed a degree of uncertainty and risk of malfunctioning. Therefore, the battery requires more testing to assess public use safety, especially in consumer electronics and the automobile industry.
Food For Thought
Sodium-sulfur batteries may be a temporary alternative to lithium-ion batteries due to their abundance in nature compared to lithium and adequate environmental friendliness. However, a shortage of sodium and sulfur is inevitable as all Earth minerals and metals are non-renewable. The world will need to capitalize on the time that the transition to sodium-sulfur batteries will buy us to search for a new, genuinely renewable solution before the environmental impact becomes irreversible—not a solution where ‘renewable’ technology relies on non-renewable resources.

